Today we will see how to make project on 'To study the various factors on which the internal resistance and emf of the cell depends' this project is only for class 12th student and this project is belongs to 'current electricity' in this project we will cover following steps
1 Introduction
2.Aim of the project
3.Theory
4.Circuit diagram
5.Apparatus Required
6. procedure followed
7.Observation
8.Result & Inferences
9.Precautions
10.Bibliography
INTRODUCTION
There is a great need of batteries in our daily use electronic appliances and the use is increasing every day. Thus , the
batteries need to be made more powerful so that their potential can be increased greatly . Thus , this project
report is based on practical analysis for the factors affecting the internal resistance of a cell. When the internal resistance of the cell is decreased we can increase the potential difference across it , and hence make it more reliable.
INTERNAL
RESISTANCE
Internal resistance is defined as the resistance offered by the electrolyte of the cell to the flow of ions.
⧭Its S.I. unit is Ohm (Ω)
⧭ For a cell of e.m.f. (E) and internal
resistance (r), connected to an external
resistance (R) such that (I) is the
current flowing through the circuit
E = V + Ir
Internal Resistance
r = E-V/I
Aim Of The Project
To study the various factors on
which the internal resistance/emf
of the cell depends .
THEORY
The internal resistance of a cell is the resistance offered by its electrolyte to the flow of ions . The internal resistance of a
cell is
directly proportional to the distance between the electrodes.
is inversely proportional to facing surface area of the electrodes in electrolyte.
decreases with increase in temperature of electrolyte.
is inversely proportional to concentration of electrolyte
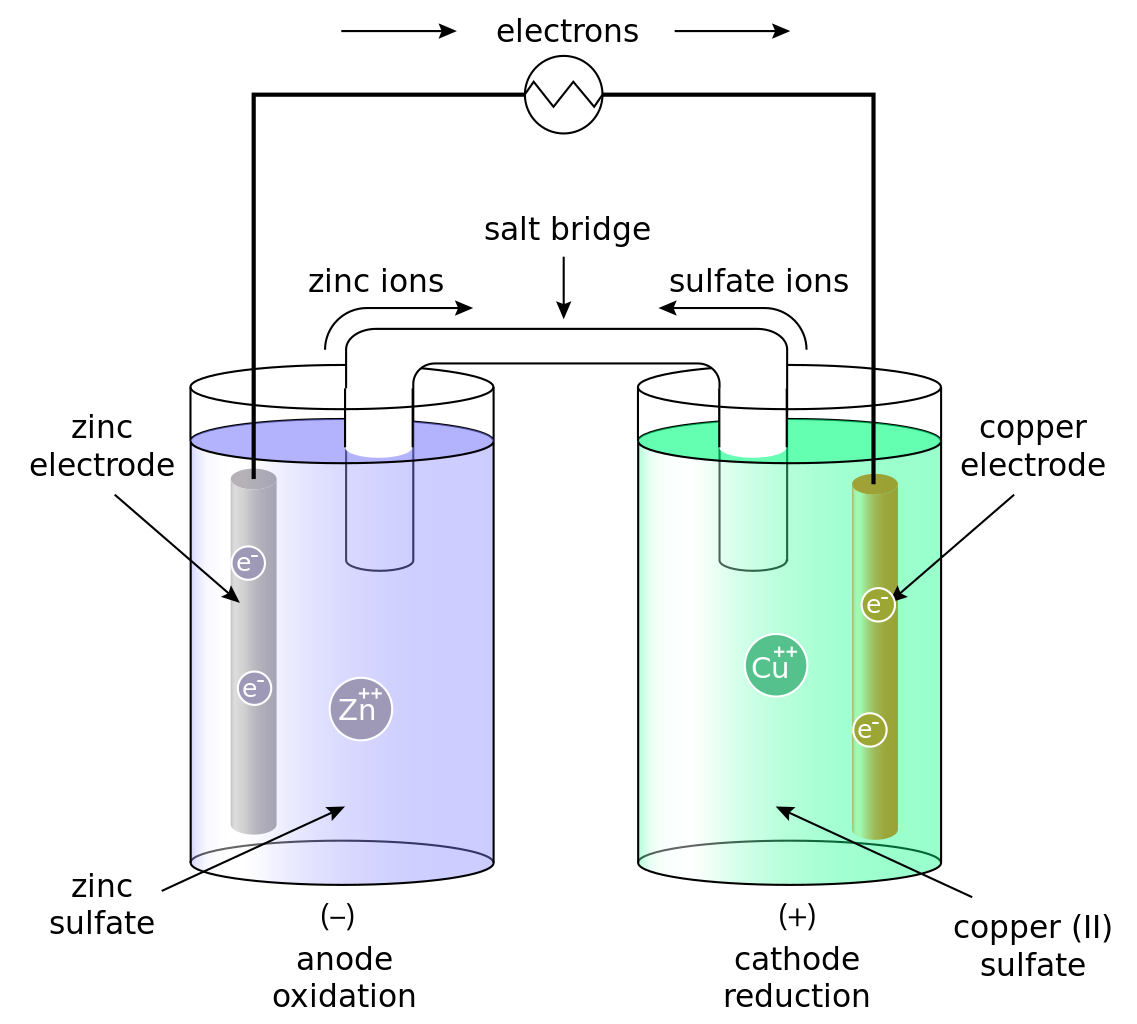
Circuit diagram
Apparatus Required
🔴 A potentiometer,
🔴a jockey,
🔴 a battery ,
🔴 three way keys,
🔴 a fractional resistance box,
🔴 a high resistance box(about 0-10000 Ω),
🔴 a rheostat of low resistance,
🔴 a voltmeter ,a primary cell(say voltaic),
🔴 electrolytes of different concentrations,
🔴 an ammeter,connecting wires and pieces of
sand paper.
Procedure Followed
1. Clean the ends of the connecting wires with sand paper and make tight connections according to the circuit diagram.
2. Tighten the plugs of the resistance box.
3. Check the e.m.f. of the battery and of the cell and make sure that e.m.f. of the battery is more than that of the cell, otherwise null or balance point will not be obtained.
To study variation of internal resistance with distance of separation
4. Keep both the electrodes at a distance of 16 cm.
5. Take maximum current from the battery, making rheostat resistance small.
6. Without inserting a plug in key K2, adjust the rheostat so that a null point is obtained on the last wire of the potentiometer.
7. Determine the position of the null point accurately using a set square and measure the balancing length (l1) between the null point and the end P.
8. Next introduce plugs in both keys K1 and K2. At the same time, take out a small resistance (1 – 5W) from the shunt resistance box connected in parallel with the cell.
9. Slide the jockey along a potentiometer wire and obtain the null point.
10. Measure the balancing length (l2) from end P. Record these observations.
11. Now keep the electrodes 12 cm apart. Then remove the plugs of keys K1 and K2. Wait for some time and repeat steps 7 to 10.
12. Next, keep the electrodes 9 cm apart to obtain another set of observations.
To study variation of internal resistance with
area of electrodes
13. Keeping all other factors constant, increase the area of electrodes in the electrolyte by dipping them into the electrolyte at different depths for each observation.
14. Obtain three such observations by repeating steps 7 to 10. Record your readings.
To study variation of internal resistance with
concentration of electrolyte
15. Keeping all other factors constant, decrease the concentration of electrolyte by adding distilled water for different observations.
16. Obtain three such observations by repeating step.
Observation
Table for effect of separation between electrodes
Table for effect of temperature
RESULT & INFERENCES
The Electromotive Force of the cell is constant and
is equal to E = 0.98 Volt.
The internal resistance of a cell is directly
proportional to the separation between the
electrodes.
The internal resistance of a cell is inversely
proportional to the area of the electrodes dipped in
electrolyte.
The internal resistance of a cell is inversely
proportional to the temperature of electrolytes.
The internal resistance of a cell is inversely
proportional to the concentration of the electrolyte.
PRECAUTIONS
1. The connections should be neat , clean and tight.
2. The plugs should be introduced in the keys only
when the observations are to be taken.
3. The positive polls of the battery E and cells E1 and E2
should , all be connected to the terminal at the zero of
the wires.
4. The jockey key should not be rubbed along the wire.
It should touch the wire gently.
5. The ammeter reading should remain constant for a
particular set of observation. If necessary , adjust the
rheostat for this purpose.
bibliography
Help from internet
1 wikipedia 2 google
Information from library
Help from teacher
NCERT textbook class 12th
NCERT physics lab manual
you can WATCH our video
you can see pdf also
Download pdf ⇒ CLICK HERE






0 Comments